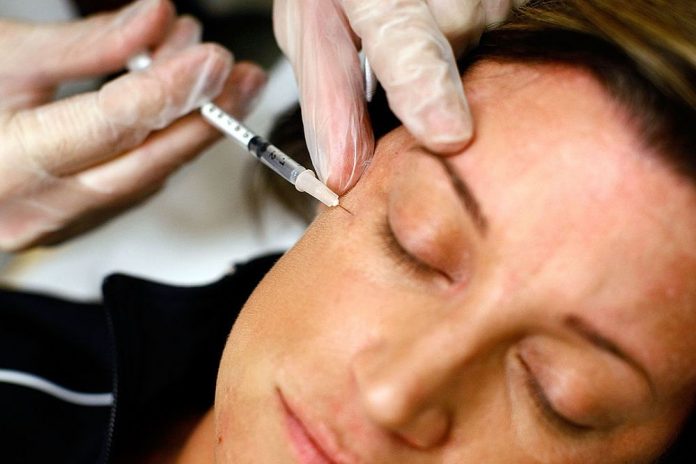
Patients are already clamoring for “the longer acting Botox,” a not-yet approved treatment from Revance Therapeutics, CEO Dan Browne told Bloomberg News in an interview Friday. The launch won’t happen for another two years or so, and while many patients want more time looking younger, doctors are looking for a longer acting product for therapeutic goals.
RT002, as the drug is known, is still in late-stage testing for its first indication — expected to be glabellar lines — results from an open-label safety study aren’t expected until the second half of this year, with a possible product launch in 2020.
“People underestimate the power of this data,” Browne said, he’s expecting it to be a “major de-risking event” for Revance when results from the study, known as “Sakura” read out later this year. Data from related late-stage studies “Sakura 1” and “Sakura 2” sent Botox-maker Allergan shares lower in December.
Revance is targeting an FDA label to show the RT002, a botulinum toxin similar to Allergan’s Botox, lasts up to six months, compared to a three to four-month label claim for Allergan’s best-selling product. The company says it has data that proves the six-month duration claim, said Chief Operating Officer Abhay Joshi, a former 18-year veteran with Allergan.
Revance sees this as a “performance product” and will seek “premium pricing,” Browne said. He plans to look at annual costs of RT002 over the cost of a single treatment. The company also announced at its investor day that it would target developing RT002 for migraines.
Companies including Biohaven and Allergan are presenting migraine data at the American Academy of Neurology meeting in L.A. this week. Revance is down 12 percent over the past two days following its first ever investor day this past Thursday.